A patient has been hospitalized with a severe case of avian influenza A(H5N1) virus (“H5N1 bird flu”) infection in Louisiana. This marks the first instance of severe illness linked to the virus in the United States. The case was confirmed by the Centers for Disease Control and Prevention (CDC) on Friday, December 13. Since April 2024, there have been a total of 61 reported human cases of H5 bird flu reported in the United States.
Partial viral genome data of the H5N1 avian influenza virus that infected the patient in Louisiana indicates that the virus belongs to the D1.1 genotype related to other D1.1 viruses recently detected in wild birds and poultry in the United States and in recent human cases in British Columbia, Canada, and Washington state. This H5N1 bird flu genotype is different than the B3.13 genotype detected in dairy cows, sporadic human cases in multiple states, and some poultry outbreaks in the United States. Additional genomic sequencing and efforts to isolate virus from clinical specimens from the patient in Louisiana are underway at CDC.
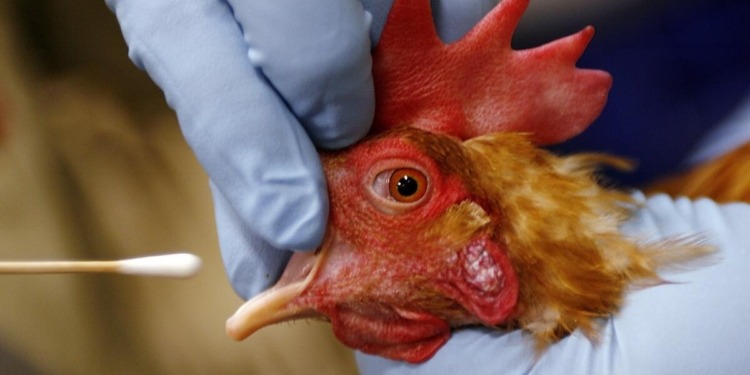
While an investigation into the source of the infection in Louisiana is ongoing, it has been determined that the patient had exposure to sick and dead birds in backyard flocks. This is the first case of H5N1 bird flu in the U.S. that has been linked to exposure to a backyard flock. A sporadic case of severe H5N1 bird flu illness in a person is not unexpected; avian influenza A(H5N1) virus infection has previously been associated with severe human illness in other countries during 2024 and prior years, including illness resulting in death. No person-to-person spread of H5 bird flu has been detected. This case does not change CDC’s overall assessment of the immediate risk to the public’s health from H5N1 bird flu, which remains low.
The H5N1 influenza virus has thus far resulted in very limited human-to-human transmission. However, it has an exceptionally high fatality rate of more than 60%, which is in sharp contrast with the 2009 H1N1 influenza virus fatality rate of around 1% (WHO 2009a).
This case underscores that, in addition to affected commercial poultry and dairy operations, wild birds and backyard flocks also can be a source of exposure. People with work or recreational exposures to infected animals are at higher risk of infection and should follow CDC’s recommended precautions when around animals that are infected or potentially infected with H5N1 avian influenza virus. This means that backyard flock owners, hunters and other bird enthusiasts should also take precautions.
The best way to prevent H5 bird flu is to avoid exposure whenever possible. Infected birds shed avian influenza A viruses in their saliva, mucous, and feces. Other infected animals may shed avian influenza A viruses in respiratory secretions and other bodily fluids (e.g., in unpasteurized cow milk or ‘raw milk’).
As a general precaution, whenever possible, people should avoid contact with sick or dead animals, in particular wild birds, and poultry.
For individuals with direct/close contact with wild birds or sick or dead poultry or other animals, wear recommended personal protective equipment (PPE). Wild birds can be infected with avian influenza A viruses even if they don’t look sick.
Do not touch surfaces or materials (e.g., animal litter or bedding material) contaminated with saliva, mucous, or animal feces from wild or domestic birds or other animals with confirmed or suspected avian influenza A virus infection.
Study of H5N1 from an infected person
An H5N1 flu virus from an infected farm worker could transmit through airborne droplets and was lethal in mice and ferrets.
The findings emphasize the risks from the current H5N1 outbreak and the need for continued monitoring and testing.
A highly pathogenic avian influenza (HPAI) H5N1 outbreak in U.S. dairy cattle began in early 2024. As of early November, infections have been confirmed in hundreds of cattle in 15 states. More than 40 human cases have also been reported. Most of the human cases were in farm workers who had mild respiratory symptoms or conjunctivitis (“pink eye”). The CDC currently considers the public to be at low risk.
A research team led by Dr. Yoshihiro Kawaoka at the University of Wisconsin-Madison previously studied an HPAI H5N1 virus found in the milk of an infected cow. That virus could infect mice and ferrets but didn’t spread efficiently through the air. The team recently did a similar study of a virus from one of the infected farm workers. Results from the study, which was funded in part by NIH, appeared in Nature on October 28, 2024.
H5N1 bird flu can be treated with flu antiviral drugs which work best when started soon after symptoms begin.
The scientists found that the virus infected and replicated efficiently in cultured human lung cells. It could also infect cultured cells from the human cornea, albeit less efficiently than in lung cells.
The virus caused lethal infections in all infected mice and ferrets. This is not unusual for an H5N1 virus. However, the cow virus studied before killed only 1 in 4 infected ferrets and required a much higher dose to kill mice. Virus was found not just in the respiratory tract, but in many tissues throughout the body, including blood, spleen, and liver.
The researchers next housed uninfected ferrets in cages next to infected ones to test whether the virus could spread through the air via respiratory droplets. They performed four separate experiments using ferrets infected with varying doses of the virus. Between 1 in 6 and 1 in 3 infected ferrets transmitted the virus to uninfected neighbors. Five of the six ferrets who became infected in this way died. This suggests that the human virus may transmit more efficiently via droplets than the cow virus, although its transmission efficiency was still limited.
FDA continues to advise against the consumption of raw milk (milk that has not been pasteurized).
The human virus contained a particular mutation that is known to promote replication in mammals. HPAI H5N1 viruses from cattle don’t have this mutation, but they do have another mutation at a nearby site. The team found that both of these mutations enhance viral replication in human cells. Notably, they also found that the cow-derived viruses were susceptible to a class of antiviral drugs called polymerase inhibitors. This suggests a potential treatment strategy for people who contract one of these viruses.
The findings support those of the earlier study suggesting that HPAI H5N1 viruses from cows could potentially transmit via respiratory droplets to infect humans. The virus from the current study only caused mild disease in the person it was isolated from. Yet its ability to cause severe disease in mice and ferrets compared to the previously studied cow virus is concerning.
Fortunately, the mutation that may contribute in part to this strain being so pathogenic hasn’t been detected in other cow viruses. “So, there are no extremely pathogenic H5N1 viruses currently circulating in cows,” Kawaoka says. “However, if a currently circulating cow H5N1 virus acquires that mutation, then that would be an issue.”
EUROPE
he risk of infection with currently circulating avian A(H5) influenza viruses of clade 2.3.4.4b in Europe remains low for the general public in the European Union/European Economic Area (EU/EEA). The risk of infection remains low-to-moderate for those occupationally or otherwise exposed to infected animals or contaminated environments.
Vaccination against influenza viruses helps to limit severe disease outcomes for people at high risk. Healthcare workers and individuals at higher risk should stay up-to-date with influenza vaccination, in accordance with national recommendations.
Several countries have made vaccination against RSV available for pregnant women and older adults, as well as immunisation with monoclonal antibodies for newborns. For more information, consult the national vaccination and immunisation recommendations made by each country’s competent authorities.


























Discussion about this post